Arrange These Solutions From Most Conductive to Least Conductive.
The atoms of metal elements are characterized by the presence of valence electrons which are electrons in the outer shell of an atom that are free to move about. Rank the elements aluminum gallium and boron in.
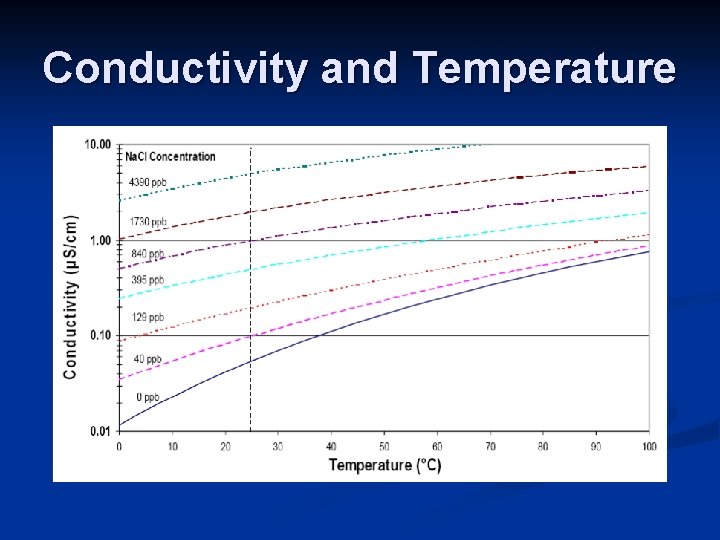
Conductivity Lecture Conductivity N A Measure Of How
The goal of this experiment is is to find the equivalence point and conductivity of six solutions 010 M NaCl 010 M LiCl 010 M KCl 010 M H3PO4 010 M C2H3O2 and 010 M HCl.

. Rank the elements aluminum sodium and phosphorus in order of decreasing conductivity. Some for their hardness others for their ductility while others are used for their resistance to corrosionMetals are also valued for their conductive properties. The most electrically conductive element is silver followed by copper and gold.
A conductor gives little resistance to the flow of electric current or heat. The graph should give you the approximate volume at. Pure silver is the most conductive of all metals.
A conductor is a material which gives very little resistance to the flow of an electric current or thermal energy. Please help me. View the answer now.
Conductivity of different ions carrying the same amount of charge can vary as per the comment of Ivan. Silver also has the highest thermal conductivity of any element and the highest light. Which lists types of materials from most conductive to least conductive.
In this question acetic acid is a weak acid so the final acetic anion will combine with the hydrogen ion from the autodissociation of water molecule to form acetic acid molecules. Electrical conductivity in metals is a result of the movement of electrically charged particles. To rank items as equivalent overlap them.
The conductivity standard of metal is based on silver which is the most conductive of all metals. Which lists the elements in order from most conductive to least conductive Get the answers you need now. View the answer now.
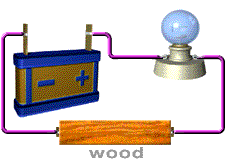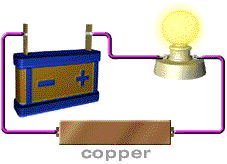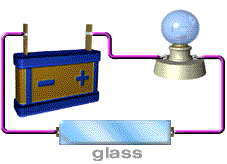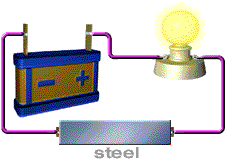
Almost no energy is lost in electricity transfer using superconductors. Rank from most conductive to least conductive. Insulator materials like rubber are the least conductive of all followed by semiconductor silicon conductor copper and the most fantastic conductors called the superconductors pure metals.
The more free electrons in a. The list of most conductive metals includes silver copper and gold. On the other hand bismuth Bi is a metal hence it will conduct electricity.
Materials are classified as metals semiconductors and insulators. This is what makes it the metal that is used in the construction of integrated circuits and in electronics whenever a path with a. Was asked on May 31 2017.
Thus we can conclude that the order from least conductive to most conductive will be nitrogen N antimony Sb bismuth Bi. It is these free electrons that allow metals to conduct an electric current. Assuming that conductivity is correlated to the number of ions in solution rank the four substances based on how well a 020 M solution in water will conduct electricity.
Conductivity is defined as the ease with which an electric charge or heat passes through a metal. Which lists the elements in order from least conductive to most conductive. The correct answer is Option A.
The most conductive pure metal is silver. Insulator semiconductor conductor superconductor. Metals are defined as the elements which loose electrons easily and thus can easily conduct electricity due to the presence of free electrons.
For a material to be a good conductor the electricity passed through it must be able to move the electrons. To rank items as equivalent overlap them. Conductivity refers to the ability of a material to transmit energy.
Heathergipson heathergipson 10142020 Chemistry. Nitrogen N antimony Sb bismuth Bi is the order from least conductive to most conductive. What valume of 025M NaOH solution is needed to react with 50ml of.
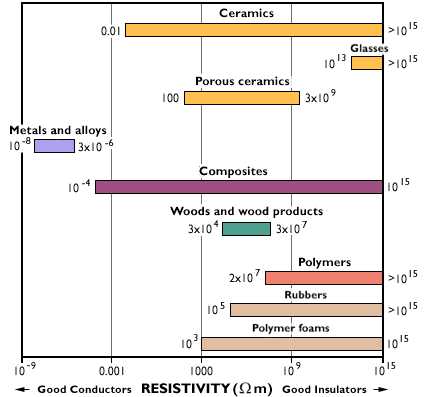
Rank from most to least conductive. Metals are the most conductive and insulators ceramics. Was asked on May 31 2017.
From the data table and graph that you created determine the volume of H2SO4 added at the equivalence point. There are different types of conductivity including electrical thermal and acoustical conductivity. Conductivity is the measure of the ease at which an electric charge or heat can pass through a material.
Eddibear3a and 6 more users found this answer helpful. Conductivity of an element is defined as the tendency to conduct electricityThis property is shown mostly by metals and then by some metalloids and least by non-metals. Thus antimony will conduct electricity.
Silver Conductivity Silver is the best conductor of electricity because it contains a higher number of movable atoms free electrons. I hope that help bye. The 5 Most Conductive Metals On Earth Are In the electroplating industry every metal serves a specific purpose.

Top 4 Most Conductive Metals Metal Men Recycling
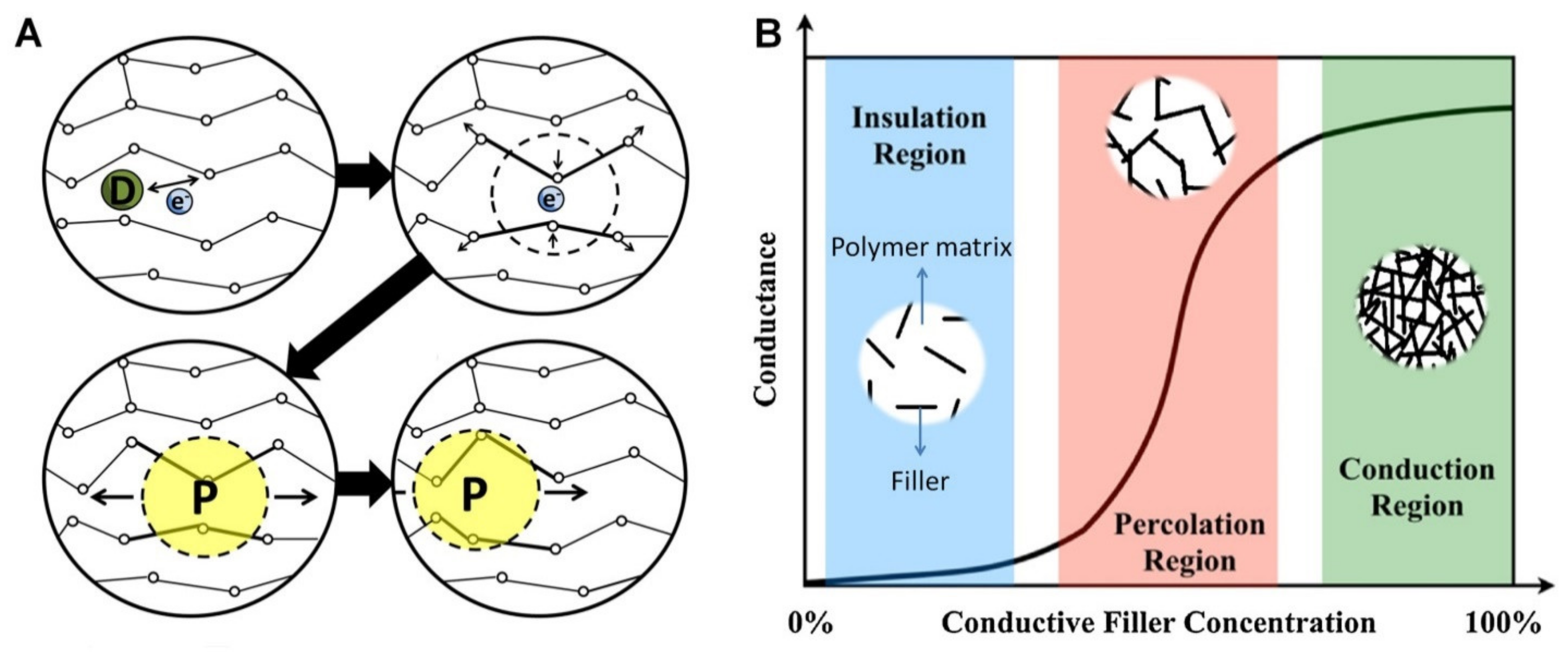
Polymers Free Full Text 3d Printable Electrically Conductive Hydrogel Scaffolds For Biomedical Applications A Review Html
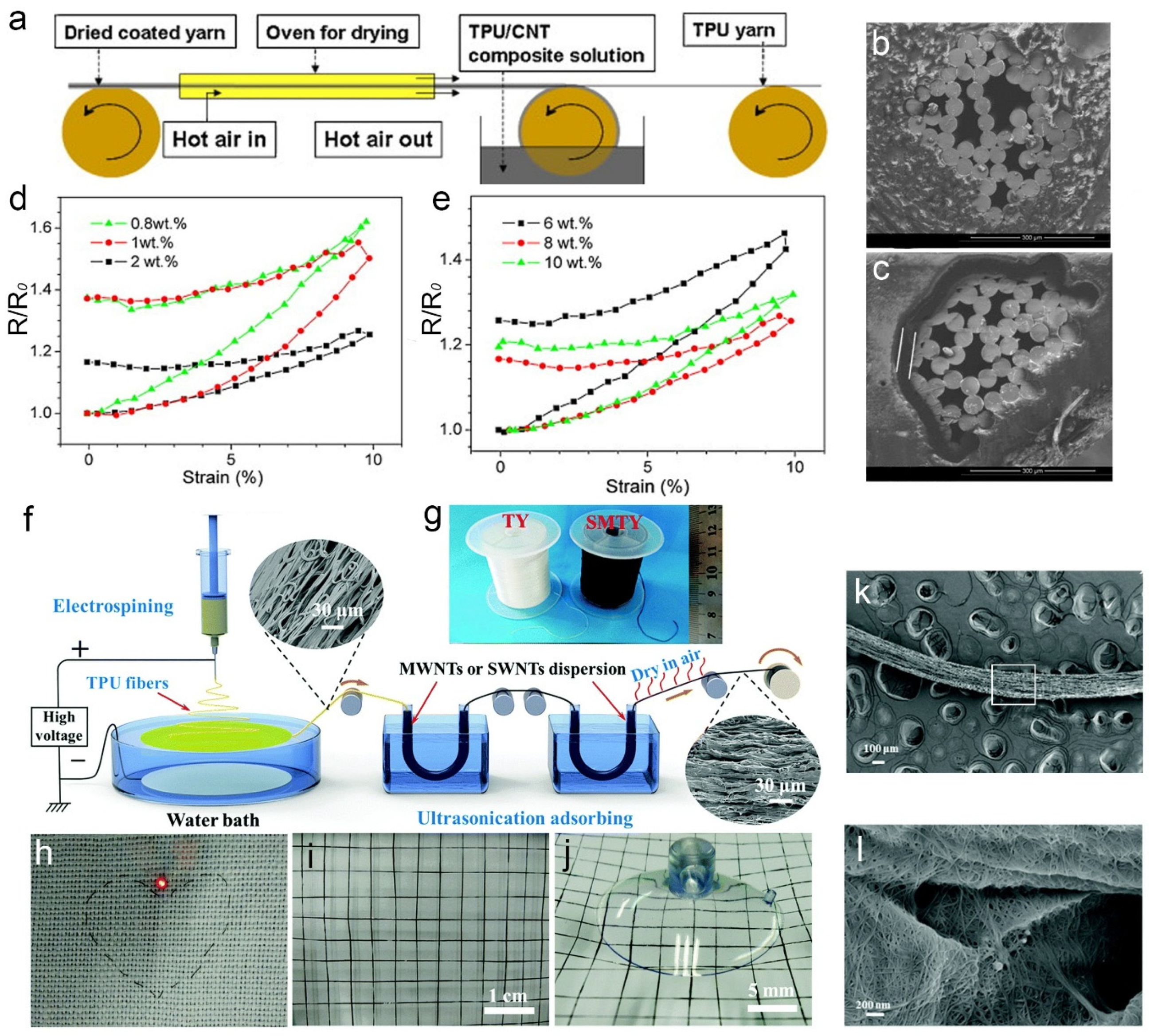
Fibers Free Full Text Electrically Conductive Coatings For Fiber Based E Textiles Html
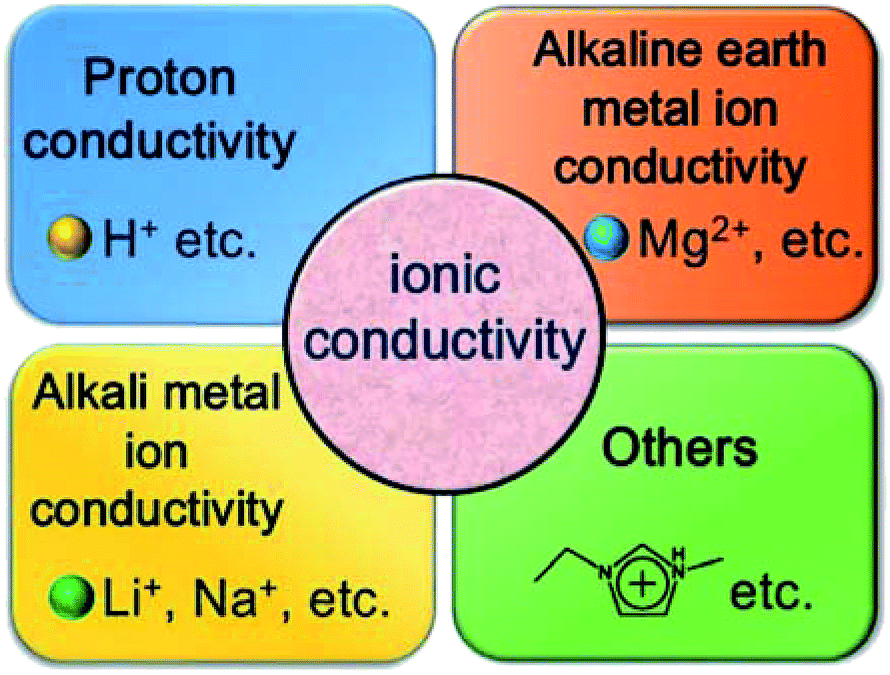
Coordination Polymer Based Conductive Materials Ionic Conductivity Vs Electronic Conductivity Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 C9ta08253k

Modular Touch Lights Hexagonal Led Wall Tile Lights With Touch Sensitive Honeycomb Magnetic Connection 10 Pack H Light Panels Wall Lights Led Night Light

Can Graphene Compete With Copper In Electrical Conductivity Bosch Global
Why Do Metals Conduct Electricity Materials Science Engineering

What Is Electrical Conductivity Know Conductivity Of Water And Saltwater Specific Conductance And Faqs
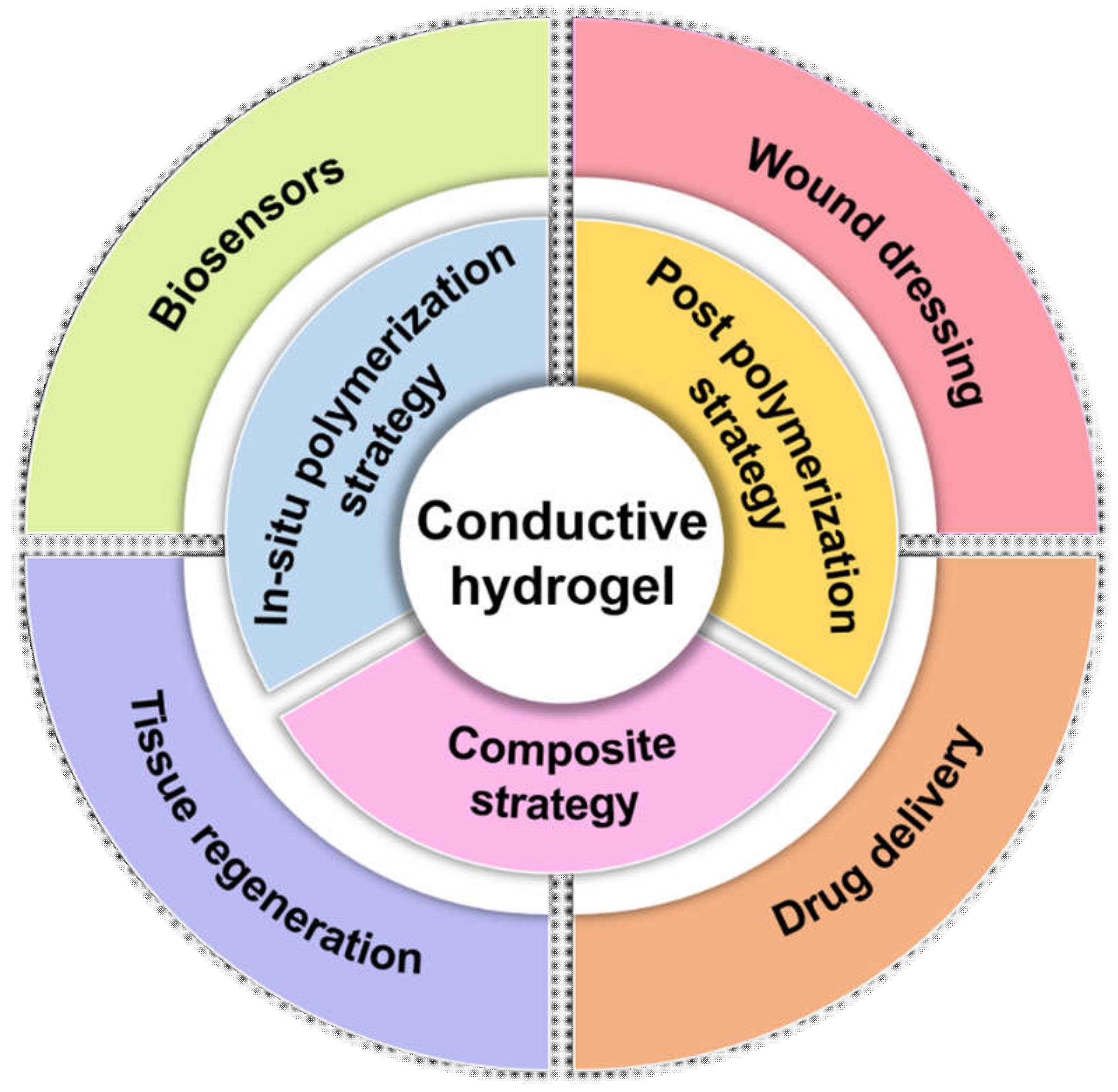
Molecules Free Full Text Design Strategies Of Conductive Hydrogel For Biomedical Applications Html
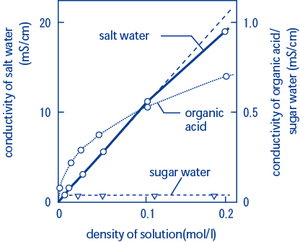
Ions In Water And Conductivity Horiba

Low Resistivity Or High Conductivity Of Conducting Material Electrical4u
Which Would Be More Conductive 0 1 M Of Nacl Or 1 0 M Of Nacl Why Quora
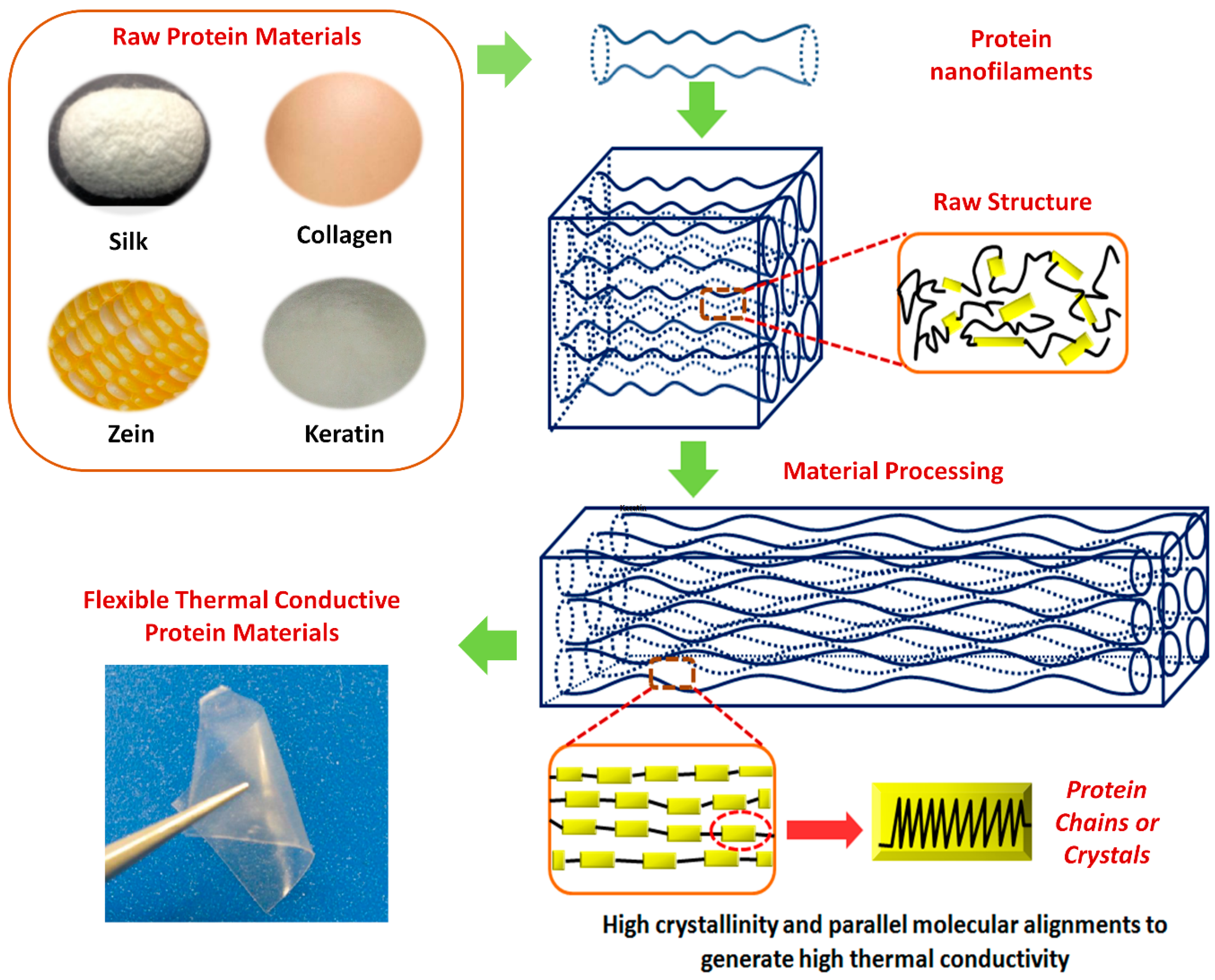
Polymers Free Full Text Thermal Conductivity Of Protein Based Materials A Review Html

Pin By Vatz Mapara On Wallpaper Shipping Paper Lithium Ion Batteries Packaging Solutions

Pdf Electrically Conductive Polymers And Composites For Biomedical Applications


Comments
Post a Comment